Training of County Super-users in readiness for implementation of TrueNat molecular platform for Tuberculosis diagnosis in Kenya
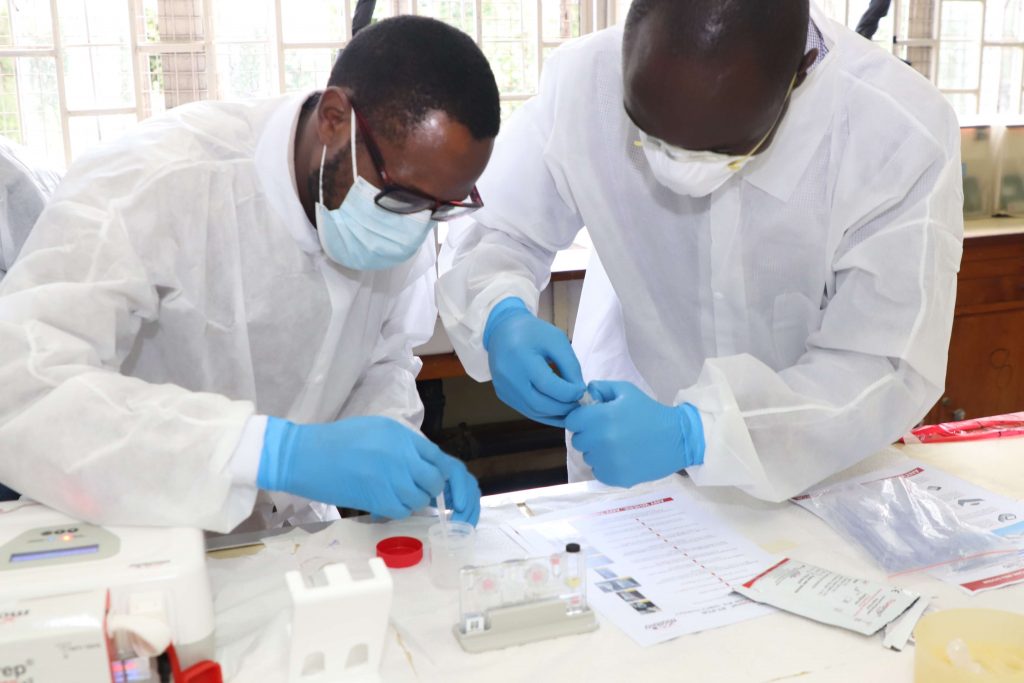
The National Tuberculosis Program (NTP) in collaboration with Infectious Diseases Detection and Surveillance (IDDs) project of USAID through ICF international is capacity-building County Truenat superusers and the end-users (Laboratory staff) from all the counties on Truenat molecular testing platform in support of Tuberculosis control.
IDDS is a USAID-funded project under the Global Health Security Agenda (GHSA) to support developing nations to strengthen detection and surveillance of priority infectious diseases like Tuberculosis, antimicrobial resistance (AMR), as well as support roll-out of innovative solutions to strengthen diagnostic networks. Under iNTP, Kenya project, IDDS will be collaborating with Centre for Health Solutions (USAID-TBARCH II-CHS-Kenya) to provide both technical and logistical assistance to NTP in the phased approach implementation of all identified activities to ensure successful completion of the project.
The training is part of the introduction of New TB Tools Project (iNTP) which is a collaboration between the Stop TB Partnership and the United States Agency for International Development (USAID) to roll-out a package of the latest innovations in diagnostics, treatments and digital health technologies.
The iNTP package of new tools include Truenat, ultra-portable digital chest X-ray machines with Computer-aided detection software (CAD), Tuberculosis (Tb) preventive therapy, Digital Adherence Technologies and Latent Tb infection testing by using IGRA/Quontiferon tests. The tools are portable equipment that will benefit TB patients as the country strives to find missing cases and end the TB epidemic in line with the declarations made during 2018 UN High-Level Meeting on TB.
Truenat is a chip-based rapid molecular test for TB that runs on the Truelab platform. The country has received 38 Truenat assay instruments which will be distributed to selected health facilities after a successful site assessment.
Truenat is designed to be operated in peripheral laboratories to be able to improve access as close to the community as possible. It is battery-powered and uses room-temperature stable reagents. It can generate results for a TB test in one hour and detection of resistance to Rifampicin in one additional hour. The results will be dispatched online to the clinicians in form of SMS and Emails using a locally developed electronic reporting platform- (Tibulims) systems to ensure that time taken to initiation on treatment is reduced.
According to Jeremiah Okari, lead iNTP coordinator at NTP, the equipment is one of the tools being implemented under iNTP, supported by the USAID in collaboration with Stop TB and GDF.
“The main objective for the iNTP is to help scale up the introduction of new WHO-approved TB molecular diagnostic tools,” he says. “This will help increase access to molecular diagnostic tools and Tuberculosis Drug Resistance surveillance, and support the rollout of treatment and digital health tools to strengthen TB care in Kenya” he adds.
Dr. Grace Kahenya, the New TB Diagnostics Tools Advisor from IDDS Head Quarter notes that the training is instrumental in supporting the country in its quest to further decentralize TB diagnosis using cutting-edge molecular innovations like Truenat which has demonstrated equivalent performance among molecular TB diagnostic tools.
Josiah Njeru, a diagnostic specialist with IDDS Kenya Country office also notes that the superuser training model has worked before in roll-out of GeneXpert. He adds that the selection and training of this level of laboratory technologists are key to support county diagnostic networks and ensure strong collaboration with the National Government in smooth implementation of diagnostic policies and guidelines.

















I want to join you soon
Congratulations to fellow Kenyans